This Fox Chase professor participates in the Undergraduate Summer Research Fellowship.
Learn more about Research Volunteering.

This Fox Chase professor participates in the Undergraduate Summer Research Fellowship.
Learn more about Research Volunteering.
Professor
Facility Director, Biological Imaging Facility
Adjunct Associate Professor, University of Pennsylvania
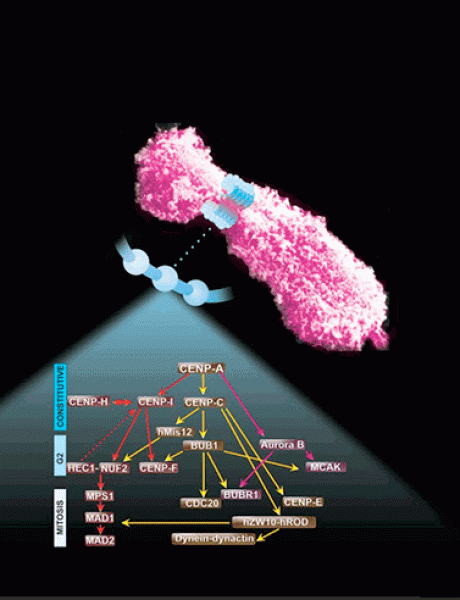
My research in fundamental mechanisms of cell division is directly relevant to understanding the biology of cancer, as well as its treatment. My focus over this time was on characterizing the kinetochore: a macromolecular structure assembled onto centromeric chromatin that specifies accurate chromosome segregation. We study the molecular functions of various proteins that reside at kinetochores as it pertains to chromosome segregation and spindle checkpoint signaling. Defects in this process lead to aneuploidy, and is, thus, of fundamental importance to problems, such as birth defects, cancer and other diseases.
We are also interested in developing new ways to enhance the efficacy of existing chemotherapies to improve treatment outcomes of cancers. In particular, we are focused on recalcitrant cancers, such as pancreatic, ovarian, brain and melanomas because the treatment outcomes are poor. Efforts to understand how these cancers survive toxic chemo and radiation therapies will reveal new targets for drug development. We, therefore, use genome-wide approches, such as RNAi and CRISPR cas9, to perform synthetic lethal screens to identify pathways that facilitate survival of cells to drugs.


Genome Stability in normal and disease states
Mancuso P., Tricarico R., Bhattacharjee V., Cosentino L., Kadariya Y., Jelinek J., Nicolas E., Einarson M., Beeharry N., Devarajan K., Katz R.A., Dorjsuren D.G., Sun H., Simeonov A., Giordano A., Testa J.R., Davidson G., Davidson I., Larue L., Sobol R.W., Yen T.J., Bellacosa A., Thymine DNA glycosylase as a novel target for melanoma. Oncogene. 38(19): 3710-3728, 2019.PMC6563616. 6.634
Sannai M., Doneddu V., Giri V., Seeholzer S., Nicolas E., Yip S.C., Bassi M.R., Mancuso P., Cortellino S., Cigliano A., Lurie R., Ding H., Chernoff J., Sobol R.W., Yen T.J., Bagella L., Bellacosa A., Modification of the base excision repair enzyme mbd4 by the small ubiquitin-like molecule sumo1. DNA Repair. 82: 102687, 2019.PMC6785017. 3.711
Bellacosa A., Yen T., Illuminating the route of precision medicine and inhibitor discovery: Real-time measurement of DNA repair capacity with molecular beacons. Oncotarget. 9(96): 36818-36819, 2018.PMC6305151. 5.168
Ghelli Luserna Di Rora A., Beeharry N., Imbrogno E., Ferrari A., Robustelli V., Righi S., Sabattini E., Verga Falzacappa M.V., Ronchini C., Testoni N., Baldazzi C., Papayannidis C., Abbenante M.C., Marconi G., Paolini S., Parisi S., Sartor C., Fontana M.C., De Matteis S., Iacobucci I., Pelicci P.G., Cavo M., Yen T.J., Martinelli G., Targeting wee1 to enhance conventional therapies for acute lymphoblastic leukemia. J Hematol Oncol. 11(1): 99, 2018.PMC6090987. 8.731
Eytan E, Wang K, Miniowitz-Shemtov S, Sitry-Shevah D, Kaisari S, et al. Disassembly of mitotic checkpoint complexes by the joint action of the AAA-ATPase TRIP13 and p31(comet). Proc Natl Acad Sci U S A. 2014 Aug 19;111(33):12019-24. PubMed PMID: 25092294; PubMed Central PMCID: PMC4142996. PubMed
Wang K, Sturt-Gillespie B, Hittle JC, Macdonald D, Chan GK, et al. Thyroid hormone receptor interacting protein 13 (TRIP13) AAA-ATPase is a novel mitotic checkpoint-silencing protein. J Biol Chem. 2014 Aug 22;289(34):23928-37. PubMed PMID: 25012665; PubMed Central PMCID: PMC4156041. PubMed
Bhattacharjee V, Zhou Y, Yen TJ. A synthetic lethal screen identifies the Vitamin D receptor as a novel gemcitabine sensitizer in pancreatic cancer cells. Cell Cycle. 2014;13(24):3839-56. PubMed PMID: 25558828. PubMed
Tipton AR, Wang K, Link L, Bellizzi JJ, Huang H, et al. BUBR1 and closed MAD2 (C-MAD2) interact directly to assemble a functional mitotic checkpoint complex. J Biol Chem. 2011 Jun 17;286(24):21173-9. PubMed PMID: 21525009; PubMed Central PMCID: PMC3122179. PubMed
Sudakin V, Chan GK, Yen TJ. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J Cell Biol. 2001 Sep 3;154(5):925-36. PubMed PMID: 11535616; PubMed Central PMCID: PMC2196190. PubMed
Chan GK, Schaar BT, Yen TJ. Characterization of the kinetochore binding domain of CENP-E reveals interactions with the kinetochore proteins CENP-F and hBUBR1. J Cell Biol. 1998 Oct 5;143(1):49-63. PubMed PMID: 9763420; PubMed Central PMCID: a.a. PubMed
Schaar BT, Chan GK, Maddox P, Salmon ED, Yen TJ. CENP-E function at kinetochores is essential for chromosome alignment. J Cell Biol. 1997 Dec 15;139(6):1373-82. PubMed PMID: 9396744; PubMed Central PMCID: PMC2132614. PubMed
Liao H, Li G, Yen TJ. Mitotic regulation of microtubule cross-linking activity of CENP-E kinetochore protein. Science. 1994 Jul 15;265(5170):394-8. PubMed PMID: 8023161. PubMed
Yen TJ, Li G, Schaar BT, Szilak I, Cleveland DW. CENP-E is a putative kinetochore motor that accumulates just before mitosis. Nature. 1992 Oct 8;359(6395):536-9. PubMed PMID: 1406971. PubMed
The following ratings and reviews are based on verified feedback collected from independently administered patient experience surveys. The ratings and comments submitted by patients reflect their own views and opinions. Patient identities are withheld to ensure confidentiality and privacy. Learn more about our Patient Experience Ratings.
This Fox Chase professor participates in the Undergraduate Summer Research Fellowship.
Learn more about Research Volunteering.




Patient comments