
PHILADELPHIA (October 30, 2020) – In a study released online today, researchers at Fox Chase Cancer Center have identified a new biomarker and potential novel treatment target for pancreatic ductal adenocarcinoma (PDAC) that could help starve cancer cells and allow the immune system to attack the tumor.
The target is a brain synaptic protein called Netrin G1 (NetG1), which was found to be ectopically expressed in the fibroblastic cells of the pancreas and also found to have a dual role in supporting PDAC survival. The role of NetG1 was discovered in a study led by Ralph Francescone, PhD, and Debora Barbosa Vendramini-Costa, PhD, postdoctoral fellows in the lab of Edna Cukierman, PhD, an associate professor in the Cancer Biology program.
The study was designed to explore the role of cancer-associated fibroblasts in PDAC growth and involved a number of researchers from various departments and programs at Fox Chase as well as collaborators from other institutions in the United States. The paper was published in Cancer Discovery, a prestigious journal of the American Association for Cancer Research.
“Cancer-associated fibroblasts reside in the organ microenvironment that is shared with the tumor cells,” said Cukierman, who is also co-director of the Marvin and Concetta Greenberg Pancreatic Cancer Institute. “Their main role is to sustain homeostatic equilibrium in the organ. If that equilibrium is broken, for example by having cancer, the fibroblastic cells expand and engage the wound repair cascade, altering the connective tissue aspect of the organ.”
Francescone and Vendramini-Costa used Cukierman’s three-dimensional culturing system, which mimics the human fibroblastic tumor microenvironment in the lab, to identify overexpression of NetG1-positive cancer-associated fibroblasts in PDAC.
One of the main functions of cancer-associated fibroblasts it to provide metabolic support to cancer cells. The study showed that NetG1 controlled glutamate and glutamine release by the cancer-associated fibroblasts, allowing cancer cells to survive in low-nutrient conditions.
“In pancreas cancer it is difficult for the cancer cells to get nutrition,” Cukierman said. “When we got rid of the NetG1 protein in the cancer-associated fibroblasts the fibroblasts looked the same but they stopped providing nutritional benefit to cancer cells.”
Cukierman and colleagues also discovered a second way that NetG1 aided in the survival of PDAC. They found that NetG1-positive cancer-associated fibroblasts are intrinsically immunosuppressive and inhibit the immune system from attacking cancer cells.
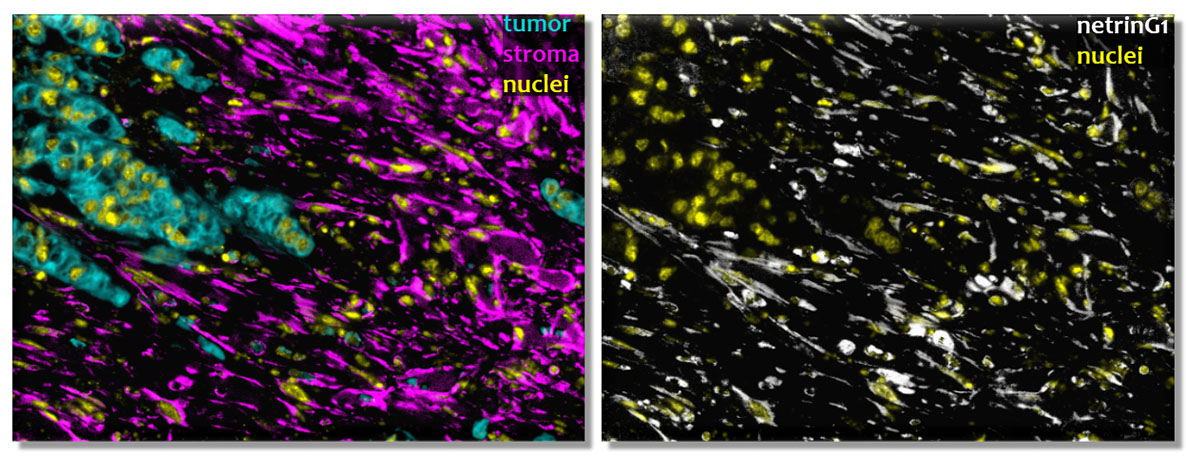
“The NetG1-positive cancer-associated fibroblasts secrete numerous cytokines, and these cells repel or suppress the immune system, basically tricking it into turning ‘off,’” Cukierman said. “We noted that when we limited the expression of NetG1 in cancer-associated fibroblasts the cells no longer secreted these immune inhibitors and actually maintained some proteins that are known to activate particular antitumor cells of the immune system.”
They found that with the lack of inhibition and the presence of activation the cancer-associated fibroblasts were good at activating natural killer cells, which attack tumors.
Based on these results, Cukierman said the presence of NetG1 can be used as a biomarker to identify a pro-tumorigenic environment that helps tumors grow.
“When a patient with pancreas cancer undergoes treatment, there can be fibrosis and necrosis because the treatment has worked, which can signify a positive outcome, but very often we are not able to differentiate between a pro-tumorigenic and an anti-tumorigenic environment because fibrosis can occur with both. NetG1 expression in cancer-associated fibroblasts could help distinguish between the two,” Cukierman said.
However, most importantly, Cukierman and colleagues were able to use an antibody to neutralize NetG1 in an animal model. This antibody helped kill the tumor and show evidence of reactivation of the immune system to effectively stall tumor progression.

“In pancreas cancer we have been hungry for something that could help us to rehabilitate the immune system to be able to kill cancer,” Cukierman said. “This paper proposes a new target to awaken the immune system while starving cancer cells at the same time.”
Click here to read the paper: “Netrin G1 Promotes Pancreatic Tumorigenesis Through Cancer Associated Fibroblast Driven Nutritional Support and Immunosuppression.”