This Fox Chase professor participates in the Undergraduate Summer Research Fellowship.
Learn more about Research Volunteering.
Related Articles
00 / 00

This Fox Chase professor participates in the Undergraduate Summer Research Fellowship.
Learn more about Research Volunteering.
Professor
Nuclear Dynamics and Cancer Program
Director, Biological Imaging Facility
Member, Cancer Epigenetics Institute










John Krais, PhD, BS
Assistant Research Professor
Room: P2101 | 215-728-7077 | [email protected]

Alice Bradbury, PhD
Postdoctoral Associate
Room: P2101 | 215-728-7133 | [email protected]

Emma Clausen, BA
Scientific Technician I
Room: P3101 | 215-278-7020 | [email protected]

Joseph Nacson, MD
Graduate Student
Room: P3101 | 215-728-3604 | [email protected]

Gregory Conway, PhD, MA
Postdoctoral Associate
Room: P2101 | 215-728-7133 | [email protected]

Pooja Patel
Scientific Technician
Room: P2101 | 215-728-7020 | [email protected]
BRCA biology and therapy resistance
The Johnson laboratory studies mechanisms of DNA damage detection, repair, and signaling that occur in BRCA1 mutation-containing organisms and cancers. We use a range of approaches, including cell biology, mouse genetics, and therapy resistance modeling, to understand basic biological processes and their implications for tumorigenesis and chemotherapy sensitivity.
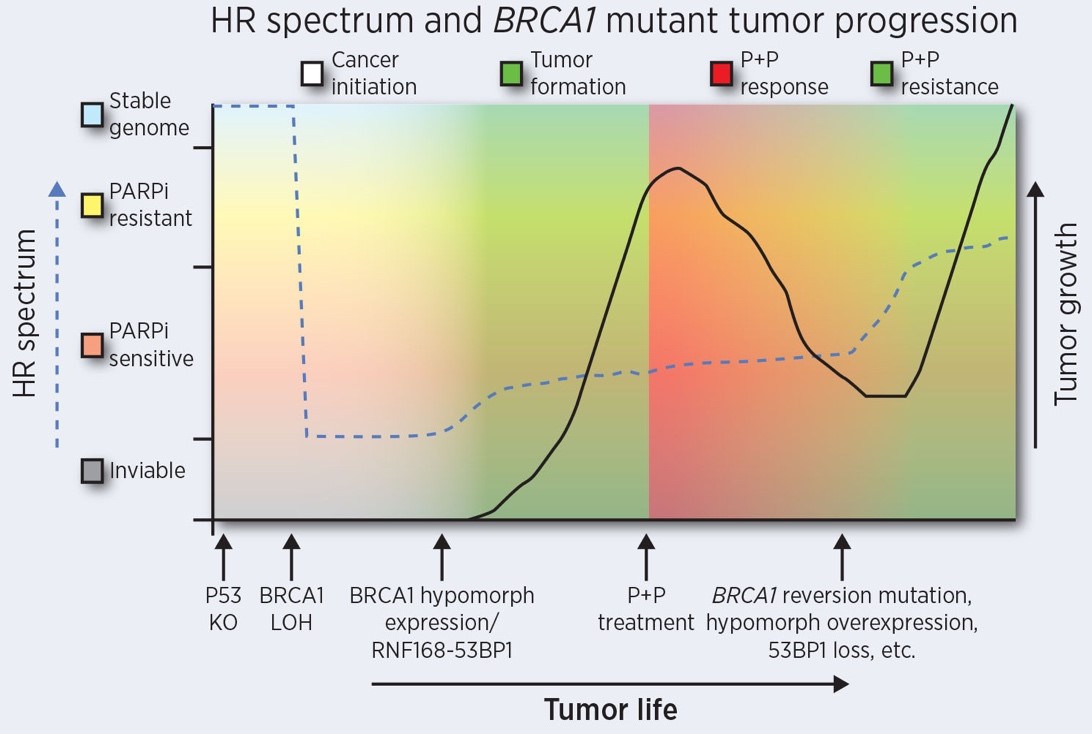
Dr. Neil Johnson is an associate professor with tenure at Fox Chase Cancer Center (FCCC) and leads an NCI R01-funded research program. Dr. Johnson’s laboratory studies mechanisms of DNA damage detection, repair, and signaling that occur in BRCA1 mutation-containing organisms and cancers. A range of approaches are routinely employed, including cell biology, mouse genetics, and therapy resistance modeling, to understand basic biological processes and their implications for tumorigenesis and chemotherapy sensitivity. In particular, Dr. Johnson’s research has focused on the utility of PARP inhibitor (PARPi) therapy for the past 10 years. Dr. Johnson used small molecule inhibitors to convert homologous recombination (HR) proficient cancer cells into HR-deficient cells, inducing ‘BRCAness’, and sensitizing cancers to PARPi. Subsequently, multiple contributions have been made toward understanding mechanisms by which BRCA1 mutation-containing alleles generate protein products that promote HR repair and chemotherapy resistance. More recently, several new BRCA1 mutant mouse alleles were generated in the Johnson laboratory to study the impact of loss of various BRCA1 functional domains on development and organismal health. Currently, the Johnson laboratory is continuing to explore DNA repair biology, and we aim to improve the current understanding of the role of BRCA1 in the repair of double stranded DNA breaks (DSBs) and maintaining genome stability. Central projects that are available to students are discussed below.
1. Determining the role of BRCA1 protein interactions in DNA repair, development and cancer.
The maintenance of genome integrity is essential for organismal development and preventing DNA damage-linked diseases such as cancer and Fanconi anemia (FA). Mutations in BRCA1 or PALB2 disrupt HR DNA repair and result in genome instability. The BRCA1 and PALB2 proteins directly heterodimerize through their respective coiled-coil (CC) domains, facilitating the formation of a larger BRCA1-PALB2-BRCA2-RAD51 complex that is required for RAD51 filament formation. BRCA1 and PALB2 form the only known interaction that is mediated by either proteins CC domain. Whether BRCA1 and PALB2 CC domains exclusively interact with one another, or there are additional CC interactions and functions is unknown. Because both BRCA1 and PALB2 form multiple protein complexes, specifically disrupting the CC domain is required to discern activities related to the BRCA1-PALB2 heterodimer. Our laboratory has developed novel BRCA1 and PALB2 CC domain mutant mouse models to investigate the significance of this interaction in DNA repair and organismal health. Brca1CC and Palb2CC alleles each have a 3-amino acid deletion in the CC domain that disrupt the BRCA1-PALB2 association but maintain protein stability and non-CC protein interactions. Collectively, we are using biochemical, cell-based, and mouse genetic experiments to evaluate the nature and importance of the BRCA1-PALB2 interaction in DNA repair and maintaining genomic integrity.
2. Identifying new biological mechanisms of BRCA1-associated DNA repair.
An accumulation of DNA breaks or large insertions and deletions can induce loss of cellular and organismal viability, as evidenced by early embryonic lethality observed in Brca1 knockout mouse models. Paradoxically, BRCA1 mutant and wild-type cancers are equally viable and malignant. To sustain viability in the absence of BRCA1, cancers require adaptations that promote the repair of endogenous DNA damage. BRCA1 mutant cancers invariably harbor TP53 mutations, and loss p53 signaling can rescue the embryonic viability of mice harboring hypomorphic Brca1 alleles. However, the embryonic viability of Brca1 null alleles cannot be rescued with Tp53 knockout. Cells harboring more deleterious BRCA1 mutant alleles may require additional epi/genetic alterations to maintain viability. In this project, we are working on novel mechanisms of DNA repair pathway re-wiring that are responsible for sustaining the viability of BRCA1 mutant cancers; with the goal of providing new insights into cellular conditions that foster genome instability and carcinogenesis.
3. Uncovering therapy response determinants in BRCA1-mutation carrying tumors.
Patients with BRCA1 mutations have better therapy response and overall survival outcomes compared to BRCA1 wild-type patients. Mutations located in exon 11 of the BRCA1 gene represent approximately 30% of the overall number of BRCA1 mutation carriers that develop cancer in the US. We recently demonstrated that BRCA1 exon 11 mutation carriers had a significantly worse overall survival compared to patients with mutations outside of this exon, and outcomes were similar to the BRCA1 wild-type patient group. Cancers with exon 11-located mutations are capable of expressing the BRCA1-Δ11q alternative splice (AS) isoform that lacks the majority of exon 11, including stop codon-inducing mutations. Currently, little is known about the ability of BRCA1-Δ11q to repair DNA damage, maintain genome integrity, and induce chemo-resistance. In this project, we are assessing the role of BRCA1-Δ11q, and the splice factors that regulate its production, in DNA repair, tumor development and therapy sensitivity. Many BRCA1 mutation-carrying patients develop therapy resistance; new biological insights gained here may result in opportunities for the design of improved prevention or treatment strategies that optimally target BRCA1 mutant cancers.
Wang Y, Bernhardy AJ, Cruz C, Krais JJ, Nacson J, Nicolas E, Peri S, van der Gulden H, van der Heijden I, O'Brien SW, Zhang Y, Harrell MI, Johnson SF, Candido Dos Reis FJ, Pharoah PD, Karlan B, Gourley C, Lambrechts D, Chenevix-Trench G, Olsson H, Benitez JJ, Greene MH, Gore M, Nussbaum R, Sadetzki S, Gayther SA, Kjaer SK; kConFab Investigators, D'Andrea AD, Shapiro GI, Wiest DL, Connolly DC, Daly MB, Swisher EM, Bouwman P, Jonkers J, Balmaña J, Serra V, Johnson N. The BRCA1-Δ11q Alternative Splice Isoform Bypasses Germline Mutations and Promotes Therapeutic Resistance to PARP Inhibition and Cisplatin. Cancer Res. 2016 May 1;76(9):2778-90. doi: 10.1158/0008-5472.CAN-16-0186 PubMed
Wang Y, Krais JJ, Bernhardy AJ, Nicolas E, Cai KQ, Harrell MI, Kim HH, George E, Swisher EM, Simpkins F, Johnson N. BRCA1 RING Domain-Deficient Proteins Promote PARP Inhibitor and Platinum Resistance. J Clin Invest. 2016 Aug 1;126(8):3145-57. PubMed
Villamar Cruz O, Prudnikova TY, Araiza-Olivera D, Perez-Plasencia C, Johnson N, Bernhardy AJ, Slifker M, Renner C, Chernoff J, Arias-Romero LE. Reduced PAK1 activity sensitizes FA/BRCA-proficient breast cancer cells to PARP inhibition. Oncotarget. 2016 Nov 22;7(47):76590-76603. doi: 10.18632/oncotarget.12576. PubMed
Johnson SF, Cruz C, Greifenberg AK, Dust S, Stover DG, Chi D, Primack B, Cao S, Bernhardy AJ, Coulson R, Lazaro JB, Kochupurakkal B, Sun H, Unitt C, Moreau LA, Sarosiek KA, Scaltriti M, Juric D, Baselga J, Richardson AL, Rodig SJ, D'Andrea AD, Balmaña J, Johnson N, Geyer M, Serra V, Lim E, Shapiro GI. CDK12 Inhibition Reverses De Novo and Acquired PARP Inhibitor Resistance in BRCA Wild-Type and Mutated Models of Triple-Negative Breast Cancer. Cell reports. 2016; 17(9):2367-2381. PubMed
George E, Kim H, Krepler C, Wenz B, Makvandi M, Tanyi JL, Brown E, Zhang R, Brafford P, Jean S, Mach RH, Lu Y, Mills GB, Herlyn M, Morgan M, Zhang X, Soslow R, Drapkin R, Johnson N, Zheng Y, Cotsarelis G, Nathanson KL, Simpkins F. A patient-derived-xenograft platform to study BRCA-deficient ovarian cancers. JCI Insight. 2017 Jan 12;2(1):e89760. doi: 10.1172/jci.insight.89760. PubMed PMID: 28097235; PubMed Central PMCID: PMC5214535. PubMed
Johnson N, Liao JB. Novel Therapeutics for Ovarian Cancer: The 11th Biennial Rivkin Center Ovarian Cancer Research Symposium. Int J Gynecol Cancer. 2017 Nov;27(9S Suppl 5):S14-S19. doi: 10.1097/IGC.0000000000001115. PubMed PMID: 29040190. PubMed
Sullivan-Reed K, Bolton-Gillespie E, Dasgupta Y, Langer S, Siciliano M, Nieborowska-Skorska M, Hanamshet K, Belyaeva EA, Bernhardy AJ, Lee J, Moore M, Zhao H, Valent P, Matlawska-Wasowska K, Müschen M, Bhatia S, Bhatia R, Johnson N, Wasik MA, Mazin AV, Skorski T. Simultaneous Targeting of PARP1 and RAD52 Triggers Dual Synthetic Lethality in BRCA-Deficient Tumor Cells. Cell Rep. 2018 Jun 12;23(11):3127-3136. doi: 10.1016/j.celrep.2018.05.034. PubMed PMID: 29898385; PubMed Central PMCID: PMC6082171. PubMed
Nacson J, Krais JJ, Bernhardy AJ, Clausen E, Feng W, Wang Y, Nicolas E, Cai KQ, Tricarico R, Hua X, DiMarcantonio D, Martinez E, Zong D, Handorf EA, Bellacosa A, Testa JR, Nussenzweig A, Gupta GP, Sykes SM, Johnson N. BRCA1 Mutation-Specific Responses to 53BP1 Loss-Induced Homologous Recombination and PARP Inhibitor Resistance. Cell Rep. 2018 Sep 25;24(13):3513-3527.e7. doi: 10.1016/j.celrep.2018.08.086. PubMed PMID: 30257212; PubMed Central PMCID: PMC6219632. PubMed
Kondrashova O, Topp M, Nesic K, Lieschke E, Ho GY, Harrell MI, Zapparoli GV, Hadley A, Holian R, Boehm E, Heong V, Sanij E, Pearson RB, Krais JJ, Johnson N, McNally O, Ananda S, Alsop K, Hutt KJ, Kaufmann SH, Lin KK, Harding TC, Traficante N, deFazio A, McNeish IA, Bowtell DD, Swisher EM, Dobrovic A, Wakefield MJ, Scott CL. Methylation of all BRCA1 copies predicts response to the PARP inhibitor rucaparib in ovarian carcinoma. Nat Commun. 2018 Sep 28;9(1):3970. doi: 10.1038/s41467-018-05564-z. PubMed PMID: 30266954; PubMed Central PMCID: PMC6162272. PubMed
Nacson J, Krais JJ, Bernhardy AJ, Clausen E, Feng W, Wang Y, Nicolas E, Cai KQ, Tricarico R, Hua X, DiMarcantonio D, Martinez E, Zong D, Handorf EA, Bellacosa A, Testa JR, Nussenzweig A, Gupta GP, Sykes SM, Johnson N. BRCA1 Mutation-Specific Responses to 53BP1 Loss-Induced Homologous Recombination and PARP Inhibitor Resistance. Cell Rep. 2018 Oct 30;25(5):1384. doi: 10.1016/j.celrep.2018.10.009. PubMed PMID: 30380426; PubMed Central PMCID: PMC6296467. PubMed
Zong D, Adam S, Wang Y, Sasanuma H, Callén E, Murga M, Day A, Kruhlak MJ, Wong N, Munro M, Ray Chaudhuri A, Karim B, Xia B, Takeda S, Johnson N, Durocher D, Nussenzweig A. BRCA1 Haploinsufficiency Is Masked by RNF168-Mediated Chromatin Ubiquitylation. Mol Cell. 2019 Mar 21;73(6):1267-1281.e7. doi: 10.1016/j.molcel.2018.12.010. Epub 2019 Jan 28. PubMed PMID: 30704900; PubMed Central PMCID: PMC6430682. PubMed
Gabbasov R, Benrubi ID, O'Brien SW, Krais JJ, Johnson N, Litwin S, Connolly DC. Targeted blockade of HSP90 impairs DNA-damage response proteins and increases the sensitivity of ovarian carcinoma cells to PARP inhibition. Cancer Biol Ther. 2019;20(7):1035-1045. doi: 10.1080/15384047.2019.1595279. Epub 2019 Mar 30. PubMed PMID: 30929564; PubMed Central PMCID: PMC6606007. PubMed
Wang Y, Bernhardy AJ, Nacson J, Krais JJ, Tan YF, Nicolas E, Radke MR, Handorf E, Llop-Guevara A, Balmaña J, Swisher EM, Serra V, Peri S, Johnson N. BRCA1 intronic Alu elements drive gene rearrangements and PARP inhibitor resistance. Nat Commun. 2019 Dec 11;10(1):5661. doi: 10.1038/s41467-019-13530-6. PubMed PMID: 31827092; PubMed Central PMCID: PMC6906494. PubMed
Park PH, Yamamoto TM, Li H, Alcivar AL, Xia B, Wang Y, Bernhardy AJ, Turner KM, Kossenkov AV, Watson ZL, Behbakht K, Casadei S, Swisher EM, Mischel PS, Johnson N, Bitler BG. Amplification of the Mutation-Carrying BRCA2 Allele Promotes RAD51 Loading and PARP Inhibitor Resistance in the Absence of Reversion Mutations. Mol. Cancer Ther. 2020 Feb;19(2):602-613. doi: 10.1158/1535-7163.MCT-17-0256. Epub 2019 Oct 1. PubMed PMID: 31575654; PubMed Central PMCID: PMC7007853. PubMed
Krais JJ, Johnson N. Ectopic RNF168 expression promotes break-induced replication-like DNA synthesis at stalled replication forks. Nucleic Acids Res. 2020 May 7;48(8):4298-4308. doi: 10.1093/nar/gkaa154. PubMed PMID: 32182354; PubMed Central PMCID: PMC7192614. PubMed
Nacson J, Di Marcantonio D, Wang Y, Bernhardy AJ, Clausen E, Hua X, Cai KQ, Martinez E, Feng W, Callén E, Wu W, Gupta GP, Testa JR, Nussenzweig A, Sykes SM, Johnson N. BRCA1 Mutational Complementation Induces Synthetic Viability. Mol Cell. 2020 Jun 4;78(5):951-959.e6. doi: 10.1016/j.molcel.2020.04.006. Epub 2020 Apr 30. PubMed PMID: 32359443; PubMed Central PMCID: PMC7418109. PubMed
Krais JJ, Wang Y, Bernhardy AJ, Clausen E, Miller JA, Cai KQ, Scott CL, Johnson N. RNF168-Mediated Ubiquitin Signaling Inhibits the Viability of BRCA1-Null Cancers. Cancer Res. 2020 Jul 1;80(13):2848-2860. doi: 10.1158/0008-5472.CAN-19-3033. Epub 2020 Mar 25. PubMed PMID: 32213544; PubMed Central PMCID: PMC7335334. PubMed
Krais JJ, Johnson N. Brca1 mutations in the coiled-coil domain impede Rad51 loading on DNA and mouse development. Mol Cell Oncol. 2020;7(5):1786345. doi: 10.1080/23723556.2020.1786345. eCollection 2020. PubMed PMID: 32944641; PubMed Central PMCID: PMC7469674. PubMed
Kim H, Xu H, George E, Hallberg D, Kumar S, Jagannathan V, Medvedev S, Kinose Y, Devins K, Verma P, Ly K, Wang Y, Greenberg RA, Schwartz L, Johnson N, Scharpf RB, Mills GB, Zhang R, Velculescu VE, Brown EJ, Simpkins F. Combining PARP with ATR inhibition overcomes PARP inhibitor and platinum resistance in ovarian cancer models. Nat Commun. 2020 Jul 24;11(1):3726. doi: 10.1038/s41467-020-17127-2. PubMed PMID: 32709856; PubMed Central PMCID: PMC7381609. PubMed
Krais JJ, Johnson N. BRCA1 Mutations in Cancer: Coordinating Deficiencies in Homologous Recombination with Tumorigenesis. Cancer Res. 2020 Aug 3;. doi: 10.1158/0008-5472.CAN-20-1830. [Epub ahead of print] Review. PubMed PMID: 32747362; NIHMSID:NIHMS1617898. PubMed Collapse
A Scientific Technician I position is immediately available in the laboratory of Dr. Neil Johnson in the Nuclear Dynamics Program, Fox Chase Cancer Center. Our research aims to understand how common cancer-causing mutations in DNA repair genes impact DNA damage signaling pathways and the response to anti-cancer therapeutics (https://www.foxchase.org/neil-johnson). Research involves mammalian tissue culture and mouse models of cancer. Candidates should be comfortable with handling and carrying out experiments involving mice.
The Scientific Technician I is an entry level technical post that contributes to the scientific research in a laboratory by performing experiments, recording results and analyzing the resultant data. The technician may also provide support to the lab by coordinating tasks, providing organizational support, training new personnel, ordering reagents and maintaining general laboratory records.
Education: Bachelors Degree: Physical or Biological Sciences (Required)
Experience: General experience and familiarity with common lab tools and equipment (Preferred) General experience with general lab techniques (Preferred)
Candidates interested in the position should send curriculum vitae, a brief description of research experience, and names of three references (Email: [email protected]).
The following ratings and reviews are based on verified feedback collected from independently administered patient experience surveys. The ratings and comments submitted by patients reflect their own views and opinions. Patient identities are withheld to ensure confidentiality and privacy. Learn more about our Patient Experience Ratings.
This Fox Chase professor participates in the Undergraduate Summer Research Fellowship.
Learn more about Research Volunteering.











Patient comments