Background
The clinical potential of poly (ADP-ribose) polymerase 1 (PARP-1) inhibitors has been increasingly recognized over the past two decades for the treatment of prostate and renal cancers. Classical PARP-1 inhibitors were designed to compete with the nicotinamide adenine dinucleotide (NAD) enzyme at the active site, providing sufficient down regulation of PARP-1-dependent genes. However, this causes inhibition of other enzymes that use NAD, including other members of the PARP family. Thus, most of the drug candidates are lack their target specificity and produce multiple off-target effects.
Summary of the Invention
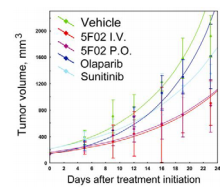
Re-evaluating cancer treatment strategies, we have developed a novel class of PARP-1 inhibitors by targeting the histone H4 route of PARP-1 activation. H4-dependent PARP-1 inhibitors demonstrate superior antitumor activity compared with the classical NAD-like PARP-1 inhibitor olaparib. Using sunitinib-resistant xenograft model of human renal cell carcinoma (RCC), we confirmed that our lead compound 5F02 provides significant antitumor activity. As demonstrated in figure below, animals treated with 5F02 showed a significant inhibition of RCC xenograft tumor growth. Notably, 5F02 revealed good oral administration. Our studies also demonstrate prominent activity of histone-dependent PARP-1 inhibitors in cell and animal models of renal, prostate and triple negative breast cancers.
Mechanism of action of novel H4-dependent PARP-1 inhibitors is completely different from that of the “classical” PARP-1 inhibitors and is unique to PARP-1. The classical PARP-1 inhibitors stabilize binding of PARP-1 to the activator histone H4 and arrest PARP-1-activator complex in transient conformation, while H4-dependent PARP-1 inhibitors disrupt this complex and exclude PARP-1 from functional complexes. Therefore, H4-dependent PARP-1 inhibitors repress transcription of PARP-1-dependent genes more effectively and specifically. In addition, H4-dependent PARP-1 inhibitors are active against PARP-1 isoform lacking catalytic domain, which is not targetable for NAD-like PARP-1 inhibitors. Novel PARP-1 inhibitors have no obvious structural homologues among components of eukaryotic enzymatic pathways, as a result minimizing the off-target effects, ensuring greater specificity and providing a new route for therapeutic applications.
Thomas C. et al. Non-NAD-Like poly(ADP-Ribose) Polymerase-1 Inhibitors effectively Eliminate Cancer in vivo. EBioMedicine. 2016 Nov;13:90-98.Opportunity: Available for licensing and/or sponsored research collaboration.
Patent Status: Published Patent Application # US 20170283402 A1
Contact:
Inna Khartchenko, M.S., MBA
Director, Technology Transfer Office of R&D Alliances
Fox Chase Cancer Center
E-mail: [email protected]
Phone: 215-214-3989